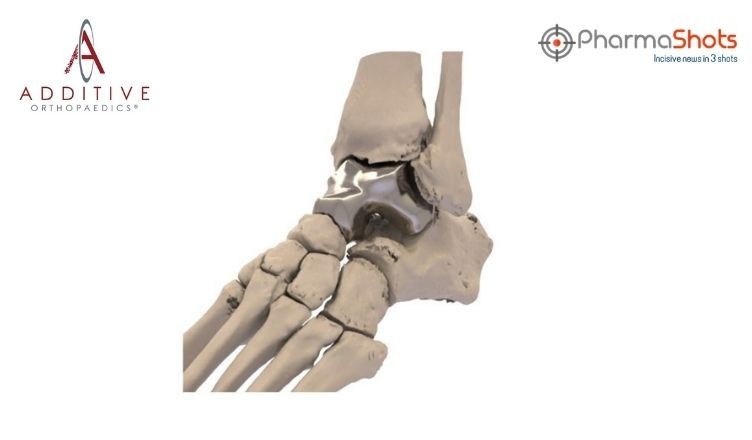
The US FDA Approves Patient Specific Talus Spacer to Treat AVN of Ankle Joint as a Humanitarian Use Device
Shots:
- The FDA has approved Patient-Specific Talus Spacer 3D-printed talus implant for humanitarian use. The FDA reviewed data through the HDE process
- Data supporting the safety of implant include results from 31 patients and 32 talus replacement surgeries with the implant. Post-operation @3yrs- pain decreased from “mod. to sev.” prior to surgery to “mild” post-surgery- and range of motion in the ankle joint is also improved
- The implant is the first of its kind to replace the talus for the treatment of AVN of ankle joint. The implant provides a joint-sparing alternative to other surgical interventions used in late-stage AVN that may disable motion of the ankle joint
Ref: FDA | Image: Crunchbase
Click here to read the full press release
Tags

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com